Igaki Lab Laboratory of Genetics, Kyoto University Graduate School of Biostudies

- Professor Tatsushi Igaki
- Research Projects
- 1. Mechanism of cell competition 2. Genetic basis of tissue growth regulation 3. Molecular basis of tumor progression and metastasis.
Cell-cell interactions in multicellular organisms play crucial roles in coordination of cell proliferation, differentiation, and cell death during development and homeostasis. However, little is known how cells communicate each other within animals to establish a multicelular system. We are exploring the molecular basis of cell-cell communication utilizing a powerful genetics of Drosophila. Especially, our research focuses on the mechanisms of cellular ‘competition’ and ‘cooperation’ within epithelium.
1) Mechanism of cell competition
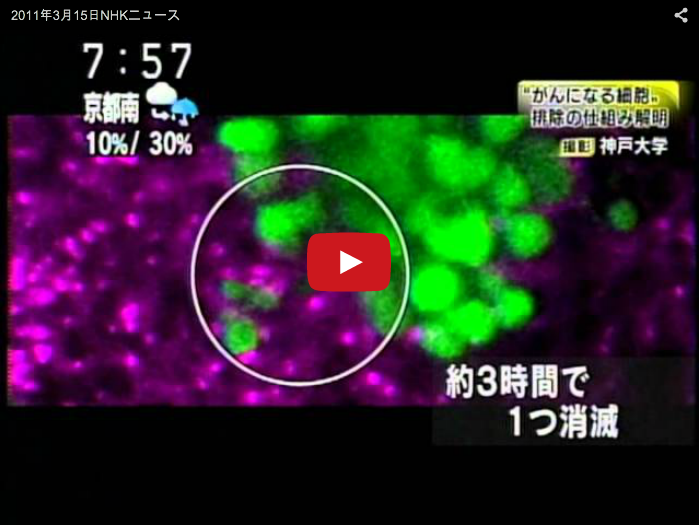
‘Cell competition’ is a form of cell-cell interaction in which cells with higher fitness (‘winners’) survive and proliferate at the expense of neighboring cells with lower fitness (‘losers’). Loser cells, but otherwise viable cells, are eliminated by cell death when confronted with winner cells. It has been suggested that cell competition is involved in a variety of biological processes such as organ size control, tissue homeostasis, cancer progression, and the maintenance of stem cell population. In developing Drosophila imaginal epithelia, clones of cells mutant for apico-basal polarity genes such as scribble (scrib) or discs large (dlg) lose their epithelial integrity and are eliminated by cell competition when confronted with wild-type cells. We have discovered that the Drosophila tumor necrosis factor (TNF) Eiger and its downstream JNK signaling play a central role in this process. Interestingly, Eiger-JNK signaling is required for both losers and winners to drive cell competition. Elevated Eiger signaling in mutant ‘loser’ cells promotes JNK-dependent cell death of these cells (Igaki et al., Dev Cell, 2009), while elevated Eiger signaling in surrounding wild-type ‘winner’ cells facilitates elimination of mutant neighbors through JNK-dependent engulfment machinery (Ohsawa et al., Dev Cell, 2011) (Fig. 1). Our study reveals that cell competition could be an evolutionarily conserved fail-safe mechanism by which animals protect against neoplastic development. To dissect the upstream mechanisms of cell competition, we have established and performed a genetic screen for genes that regulate this cell elimination. We have also established new models of cell competition using different types of mutations to understand the molecular mechanism and the physiological roles of cell competition.
2) Mechanism of tissue growth and tumor progression through cell-cell communication
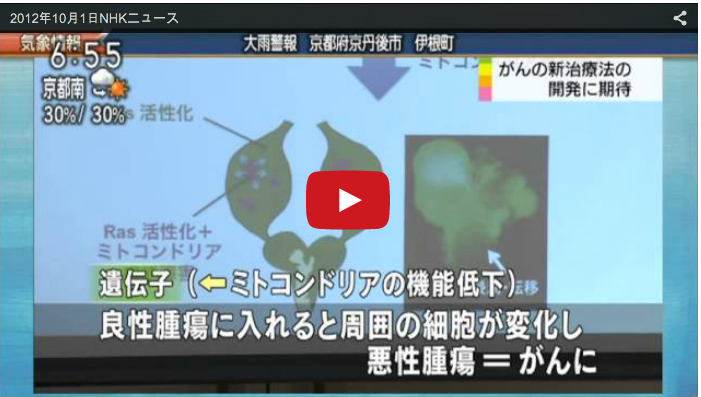
Cell-cell interactions between oncogenic cells and surrounding normal cells in the tumor microenvironment play crucial roles in cancer progression. However, the mechanisms by which each oncogenic alteration cooperates with others to drive tissue growth and tumor progression through cell-cell communication remain elusive. We have been studying the mechanism of tumor growth and metastasis using the Drosophila model of tumor progression (Igaki et al., Curr Biol, 2006). Furthermore, we have performed a genetic screen in Drosophila imaginal epithelium to identify mutations that cause ‘non-autonomous’ tumor progression through cell-cell communication. The results from our screen revealed that defects in mitochondrial respiratory function in conjunction with Ras activation potently induce tumor progression of surrounding tissue. Mechanistically, Ras activation and mitochondrial dysfunction cooperatively stimulate production of ROS, which causes activation of JNK signaling. JNK cooperates with oncogenic Ras to inactivate the Hippo pathway, leading to upregulation of the inflammatory cytokine Unpaired (Upd, an IL-6 homolog). The secreted Upd further cooperates with Ras signaling in neighboring cells with normal mitochondrial function, causing benign tumors to exhibit metastatic behavior (Ohsawa et al., Nature, 2012) (Fig. 2). These findings provide a novel mechanistic basis for interclonal tumor progression driven by ‘oncogenic inflammation’ through Ras activation and mitochondrial dysfunction, the frequent alterations in human malignancies. We have also discovered that oncogenic cells with elevated Src activity promote growth of surrounding tissue via JNK-dependent regulation of the Hippo pathway (Enomoto and Igaki, EMBO Rep, 2012). We are also establishing new models of cellular ‘cooperation’ that regulate tissue growth and/or tumor progression through cell-cell communications.
- Selected publications:
- *Nakamura M, *Ohsawa S, Igaki T "Mitochondrial defects trigger proliferation of neighbouring cells via a senescence-associated secretory phenotype in Drosophila" Nature Communications Oct27 ;5:5264 (*equal contribution)(2014) [PubMed]
- Enomoto M, Igaki T "Src controls tumorigenesis via JNK-dependent regulation of the Hippo pathway in Drosophila" EMBO Reports 14,65-72 (2013) [PubMed]
- Ohsawa S, Sato Y, Enomoto M, Nakamura M, Betsumiya A, Igaki T. "Mitochondrial defect drives non-autonomous tumor progression through Hippo signaling in Drosophila" Nature 490, 547-551 (2012) [PubMed]
- Ohsawa S, Sugimura K, Takino K, Xu T, Miyawaki A, Igaki T. "Elimination of oncogenic neighbors by JNK-mediated engulfment in Drosophila" Developmental Cell 20, 315-328 (2011) [PubMed]
- Lab meetings:
- Progress report (Friday, 10:00am; English) Journal club (Friday, 9:00am; Japanese) One on one (or small group) meeting (3:00pm- once in three weeks; English or Japanese)
Nagata R, Akai N, Kondo S, Saito K, Ohsawa S and Igaki T.
"Yorki drives supercompetion by non-autonomous induction of autophagy via bantam microRNA in Drosophila"
Current Biology 2022 Feb 07 [PubMed]
Ochi N, Nakamura M, Nagata R, Wakasa N, Nakano R and Igaki T.
"Cell competition is driven by Xrp1-mediated phosphorylation of eukaryotic initiation factor 2α"
PLOS Genetics 2021 Dec 6;17(12):e1009958 [PubMed]
Cong B, Nakamura M, Sando Y, Kondo T, Ohsawa S and Igaki T.
"JNK and Yorkie drive tumor malignancy by inducing L-amino acid transporter 1 in Drosophila"
PLOS Genetics 2021 Nov 15;17(11):e1009893 [PubMed]
#Enomoto M, Takemoto D and #Igaki T.
"Interaction between Ras and Src clones causes interdependent tumor malignancy via Notch signaling in Drosophila"
Developmental Cell 2021 Aug 9;56(15):2223-2236.e5. [PubMed]
Wada Y, Ohsawa S and Igaki T.
"Yorkie ensures robust tissue growth in Drosophila ribosomal protein mutants"
Development 2021 Jul 6;dev.198705.[PubMed]
Ito T and Igaki T.
"Yorkie drives Ras-induced tumor progression by microRNA-mediated inhibition of cellular senescence"
Science Signaling 2021 Jun 1;14(685), eaaz3578 [PubMed]
*Akai N, *#Ohsawa S, Sando Y and #Igaki T.
"Epithelial cell turnover ensures robust coordination of tissue growth in Drosophila ribosomal protein mutants"
PLOS Genetics 2021 Jan 28;17(1):e1009300 [PubMed]
Sanaki Y, Nagata R, Kizawa D, Léopold P and Igaki T.
"Hyperinsulinemia drives epithelial tumorigenesis by abrogating cell competition"
Developmental Cell 2020 May 18;53(4):379-389 [PubMed]
Nagata R, Nakamura M, Sanaki Y and Igaki T. "Cell competition is driven by autophagy" Developmental Cell 2019 [PubMed]
Katsukawa M, Ohsawa S and Igaki T. "Serpin Facilitates Tumor-Suppressive Cell Competition by Blocking Toll-Mediated Yki Activation in Drosophila" Current Biology 2018 May 15 [PubMed]
Ohsawa S, Vaughen J and Igaki T. "Cell Extrusion: A Stress-Responsive Force for Good or Evil in Epithelial Homeostasis." Developmental Cell 2018 Feb 5;44 (3):284-296 [PubMed]
Vaughen J and Igaki T. "Breaking Down Neighbors to Fuel Tumorigenesis" Developmental Cell 2017 Feb 6;40 (3):219-220 [PubMed]
*Yamamoto M, *Ohsawa S, Kunimasa K and Igaki T. "The ligand Sas and its receptor PTP10D drive tumor-suppressive cell competition" Nature 2017 Feb 9;542 (7640):246-250 (*equal contribution) [PubMed]
Vaughen J and Igaki T. "Slit-Robo Repulsive Signaling Extrudes Tumorigenic Cells from Epithelia" Developmental Cell 2016 Dec 19;39 (6):683-695 [PubMed]