原理を理解する
![]() Understanding the translocation mechanism of importin beta through the Nuclear Pore Complex
Understanding the translocation mechanism of importin beta through the Nuclear Pore Complex
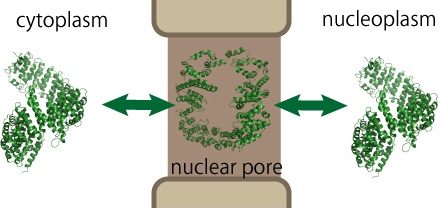
Macromolecular traffic through the nuclear pore complex (NPC) regulates communication between cytoplasm and nucleoplasm and determines the intracellular distribution of cellular proteins. Karyopherins mediate the transport of a number of proteins beyond the size barrier of 40 kDa. In spite of the low sequence conservation among karyopherins, their tertiary structures are similar; they are composed of a number of amphiphilic HEAT motifs.
We are focusing on the fact that these HEAT motifs are composed of amphiphilic a-helices, and trying to elucidate a relationship between structural flexibility of HEAT repeats and transport ability through the NPC. Since the central channel of the NPC is rich in hydrophobic side chains, conformational changes in amphiphilic motifs seem to be suitable for passing through such a hydrophobic environment.
To elucidate the structural changes of importin beta during the passage through the NPC, we utilize the following approaches.
i) Biochemical approaches to see global conformational changes
ii) Spectroscopic approaches to see secondary and tertiary structures
iii) Computational simulation to see conformational dynamics at atomic level