Member
|
KAI, ToshieProfessor |
kai.toshie.2w*kyoto-u.ac.jp |
|---|---|
|
USUI, TadaoJunior Associate Professor |
usui.tadao.3c*kyoto-u.ac.jp See faculty information |
- Please note that the @ symbol has been replaced by *.
Access
Faculty of Medicine Campus, Faculty of Medicine Bldg. G South Campus Research Bldg.
Kai Group research summary
Research outline
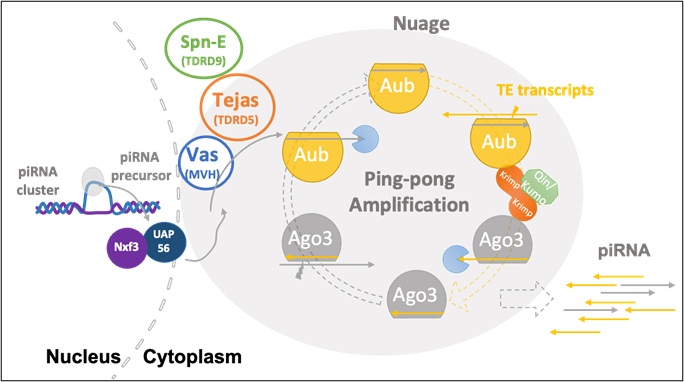
Higher animals are mortal as individuals, but species survive through sexual reproduction. In this view, individuals are transient carriers of genetic information, while germline cells are essential for species continuity. Using Drosophila melanogaster as a model, we study how germline stem cells are maintained in niches within ovaries and testes, and how they differentiate into eggs or sperm. In addition, we also focus on piRNAs, small non-coding RNAs expressed in the germline. piRNAs suppress transposable elements and protect genome integrity. They are produced in a non-membranous organelle called nuage, which serves as a platform for piRNA biogenesis. Disruption of nuage impairs piRNA production, leading to genome instability, defective gametes, and infertility. We use genetics, cell biology, bioinformatics, and AlphaFold-based protein structure prediction to investigate the formation and function of nuage and piRNAs, and their role in germline development.
Main themes
・Mechanisms of germline stem cell maintenance and differentiation into eggs and sperm
・Biogenesis of piRNAs and their function in transposon silencing in the germline
・Structural and functional analysis of nuage and its role in piRNA production
Usui Group research summary
Research outline
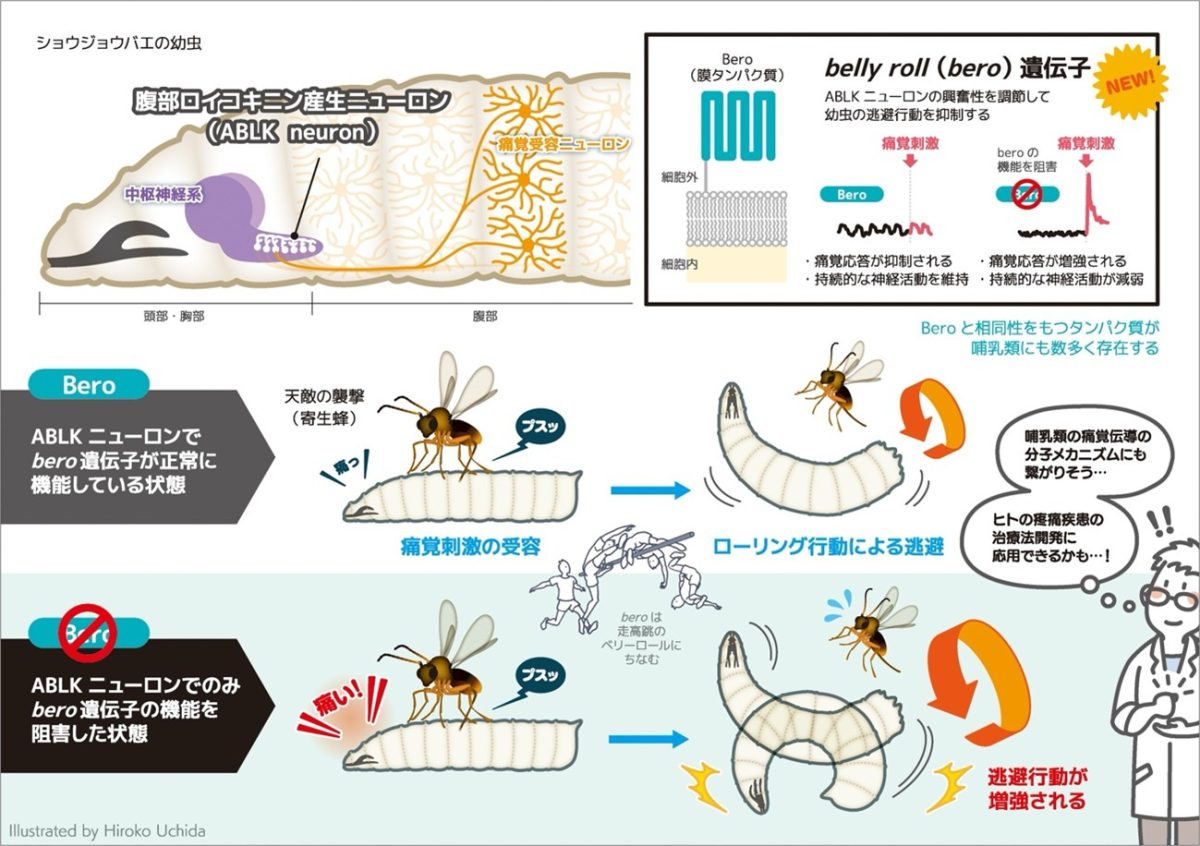
To understand the neural mechanisms that regulate the nociceptive escape behaviors, we compared larval behaviors of wild-type strains of Drosophila (Drosophila melanogaster Genetic Reference Panel; Mackay et al., Nature, 2012). A genome-wide search for single nucleotide polymorphisms (SNPs) that strongly correlate with the temporal patterns of the escape behavior has successfully identified a number of candidate genes. Among them, we are focusing on belly roll (bero), a novel gene that suppresses the escape behavior, for further investigation.
The bero gene encodes a GPI-anchored membrane protein that is expressed in several types of neuropeptide-positive neurons in the central nervous system. increased the response to pain input and enhanced escape behavior. Furthermore, inducing neural activity specifically in ABLK neurons using optogenetics induced nociceptive escape behavior (Li et al., eLife, 2023). In other words, the Bero protein expressed in ABLK neurons may be involved in the regulation of escape behavior by inhibiting the ABLK neurons’ own response to painful stimuli.
Interestingly, ABLK neurons show spontaneous neural activity even before receiving painful stimuli, and this spontaneous activity can be assumed to reflect some sensory information other than painful input. We have proposed the hypothesis that ABLK neurons are neurons that modulate nociceptive transmission by internal environmental factors through the function of Bero protein. Further analysis is currently underway to test this hypothesis and to understand the physiological mechanisms.
Main themes
・Search for genes regulating nociceptive escape behavior in Drosophila larvae
・Environment-dependent regulation of innate behavior in Drosophila larvae
・Pursuit of environmental adaptation mechanisms in Drosophilidae